P4
Hans-Georg Koch / Petra Hellwig (Strasbourg)
Assembly of cytochrome cbb3-type Cytochrome oxidases
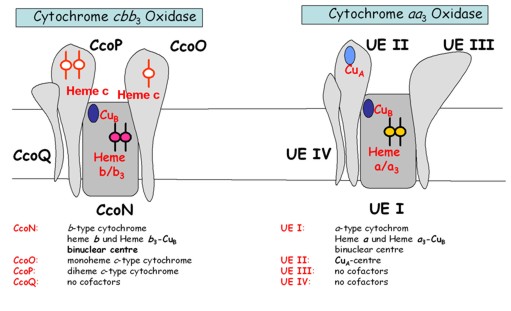
Heme-copper oxidases are universally conserved integral multisubunit membrane proteins that catalyze the terminal reaction of respiratory electron transport chains in mitochondria and aerobic bacteria. Bacterial respiratory chains usually contain multiple terminal oxidases, which allow bacteria to adapt to different environmental O2 concentrations during their life cycle. The ability to cope with low oxygen concentrations is in particular important for pathogenic bacteria, which colonize human tissues of low oxygen concentration, like the intestine. Many of these bacteria like Helicobacter pylori, Vibrio cholerae or Campylobacter jejuni, contain a special kind of heme-copper oxidase, the cbb3-type Cytochrome oxidase. The major advantage of this enzyme is that its oxygen affinity is significantly higher than the oxygen affinity of the mitochondrial-type aa3-type Cytochrome oxidase. The frequent occurrence of cbb3-type oxidases in pathogenic bacteria has raised the question of whether cbb3-type oxidases and their specific assembly factors are important determinants of pathogenicity.
The characteristic subunit composition and cofactor content of cbb3 type oxidases in comparison to aa3 type oxidases, suggests a specific assembly pathway with dedicated assembly factors. The temporal and spatial dissection of the cbb3 oxidase assembly pathway offers the opportunity to specifically interfere with cbb3 oxidase assembly without affecting the aa3 oxidase in the human host. Indeed, we have identified several assembly proteins which are required for cbb3-type oxidase assembly, but which are not involved in aa3 type oxidase assembly. The exact function of these assembly factors need to be determined at a molecular level and their potential to develop into a drug target need to be explored.
We employ a wide range of highly relevant and sophisticated methods, including: in vitro transcription/translation systems; chemical and site directed cross-linking; BN-PAGE and 2D-PAGE; Spectroscopic analyses (in collaboration with P. Hellwig, University Straßburg); in vivo FRET analyses (in collaboration with P. Graumann, University Freiburg & I. Schalk, ESBS Straßburg)
Selected Publications:
Kulajta, C., Thumfart, J., Haid, S., Daldal, F. and Koch, H.-G. (2006). Multi-step assembly pathway of the cbb3-type cytochrome c oxidase complex. J. Mol. Biol. 355, 989-1004.
Aygun-Sunar, S., Mandaci, S., Koch, H.-G., Murray, I.V.J., Goldfine, H. and Daldal, F. (2006). Ornithine lipid is required for optimal steady-state amounts of c-type cytochromes in Rhodobacter capsulatus. Mol. Microbiol. 61, 418-435.
Koch, H.-G. and Schneider, D (2007) Folding, assembly and stability of transmembrane cytochromes. Current Chemical Biology 1; 59-74
Sanders, C., Turkarsalan, S., Onder, O., Frawley, E.R., Kranz, R.G., Koch, H.-G. and Daldal, F., (2008) Biogenesis of c-type cytochromes and cytochrome complexes. In: Advances in Photosynthesis and Respiration (Govindjee, Hrsg), Springer Verlag, Berlin, in press