P2
Thorsten Friedrich
Structure and function of the NADH:ubiquinone oxidoreductase
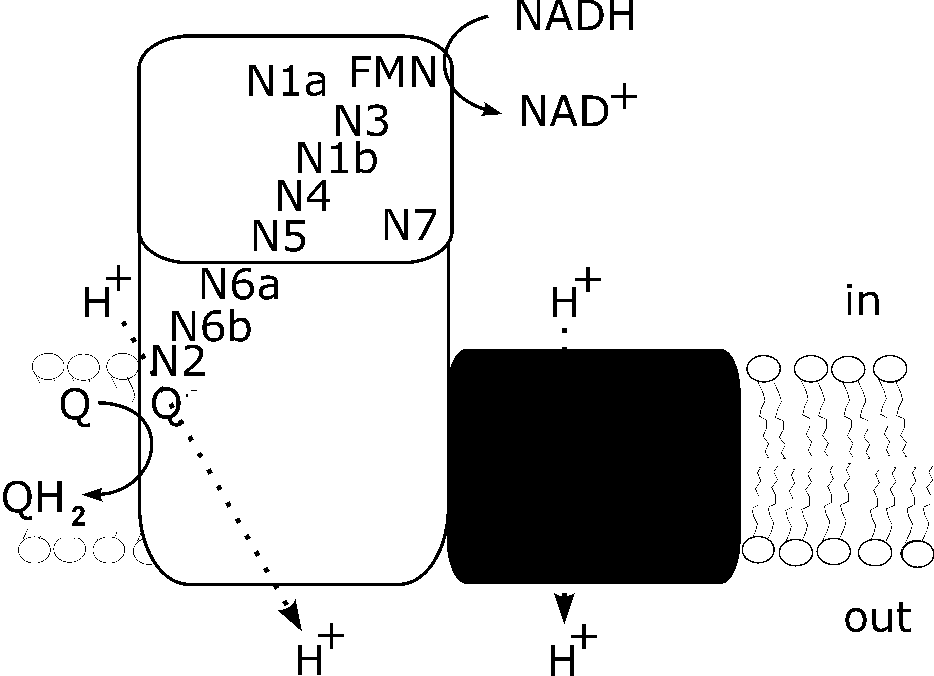
The proton-pumping NADH:ubiquinone oxidoreductase, also known as respiratory complex I, links the transfer of electrons from NADH to ubiquinone with the translocation of protons across the membrane. In doing so, complex I establishes a proton motive force required for energy consuming processes like ATP-synthesis. While the mechanism of the other enzyme complexes of the respiratory chains are discussed on a molecular level, little is known about the mechanism of complex I. Electron microscopy revealed the unusual ‘L’-shape of the complex consisting of two arms arranged perpendicular to each other (Fig. 1). The so-called peripheral arm protrudes into the aqueous phase while the so-called membrane arm is buried in the lipid bilayer. Recently, the structure of the peripheral arm was solved to molecular resolution. Phylogenetic analysis revealed the evolution of complex I from an NADH dehydrogenase module, a hydrogenase module and a transporter module (Fig. 1).
Fig. 1: Model of the E. coli complex I. N1a to N7 represent the Fe/S cluster, Q, ubiquinone. The NADH dehydrogenase module is shown in white, the hydrogenase module in grey, and the transporter module in black.
The aim of this project is to gain insight into the structure of complex I and its modules through a combination of genetic, biochemical and biophysical approaches. The structure will be determined by electron microscopy and X-ray analyses. The effects of lipids on the structure and function of the E. coli complex I will be investigated addressing the specifity and the molecular mechanism(s) of the interaction between the lipids and the enzyme.